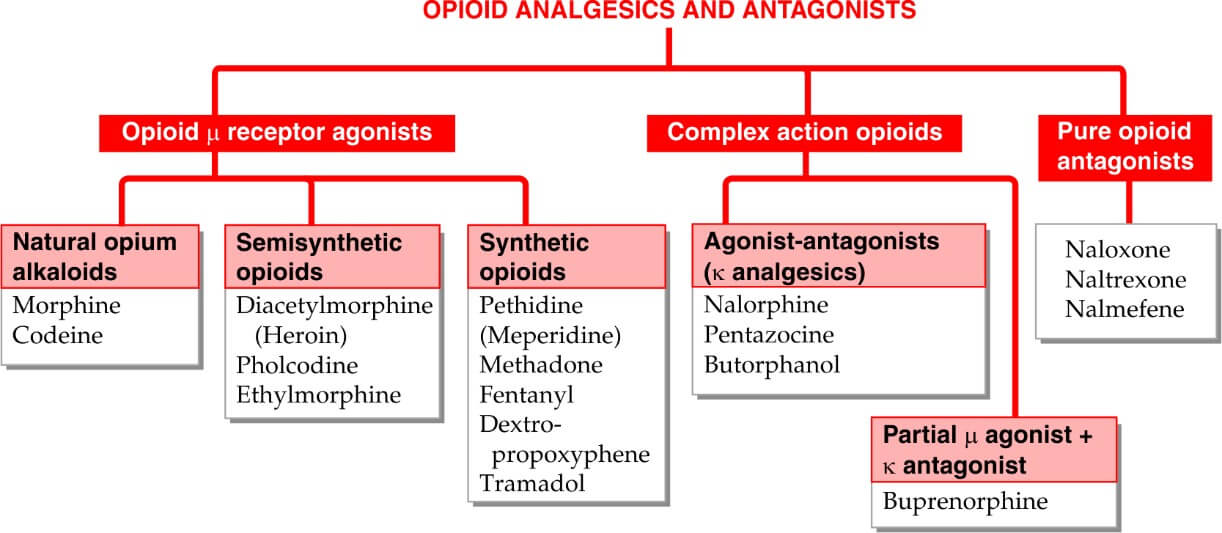
Table of Contents
Classifications
Pain
Pain refers to an unpleasant sensory and emotional experience associated with actual or potential tissue damage. The word pain has been derived from a Greek word Poena meaning ‘penalty or punishment’.
BENEFITS OF PAIN SENSATION
Pain is an important sensory symptom. Though it is an unpleasant sensation, it has protective or survival benefits such as:
- Pain gives warning signal about the existence of a problem or threat. It also creates awareness of injury.
- Pain prevents further damage by causing reflex withdrawal of the body from the source of injury
- Pain forces the person to rest or to minimize the activities thus enabling rapid healing of injured part
- Pain urges the person to take required treatment to prevent major damage.
COMPONENTS OF PAIN SENSATION
Pain sensation has two components:
- Fast pain
- Slow pain
Fast pain is the first sensation whenever a pain stimulus is applied. It is experienced as a bright, sharp and localized pain sensation. Fast pain is followed by the slow pain, which is experienced as a dull, diffused and unpleasant pain. Receptors for both the components of pain are same, i.e. the free nerve endings. But, afferent nerve fibers are different. Fast pain sensation is carried by A δ fibers and slow pain sensation is carried by C type of nerve fibers.
PATHWAYS OF PAIN SENSATION

Pain sensation from various parts of body is carried to brain by pathway:
Nociceptors
Nociceptor is the name given to receptors of pain to indicate that they respond to noxious stimuli. Receptors of pain sensation are the free nerve endings, which are distributed throughout the body.
The noxious stimuli can be damaging or potentially damaging mechanical, chemical and thermal stimuli.
Structure- Nociceptors refer to special type of free nerve endings of two types of nerve fibers:
- Aδ myelinated nerve fibers
- C unmyelinated nerve fibers.
First Order Neurons
First order neurons are the cells in posterior nerve root ganglia, which receive the impulses of pain sensation from pain receptors through their dendrites. These impulses are transmitted to spinal cord through the axons of these neurons.
Fast pain fibers
Fast pain sensation is carried by Aδ type afferent fibers which synapse with neurons of marginal nucleus in the posterior gray horn.
Slow pain fibers
Slow pain sensation is carried by C type afferent fibers, which synapse with neurons of substantia gelatinosa of Rolando in the posterior gray horn.
Second Order Neurons
Neurons of marginal nucleus and substantia gelatinosa of Rolando form the second order neurons. Fibers from these neurons ascend in the form of the lateral spinothalamic tract.
Fast pain fibers
Fibers of fast pain arise from neurons of marginal nucleus. Immediately after taking origin, the fibers cross the midline via anterior gray commissure, reach the lateral white column of the opposite side and ascend. These fibers form the neospinothalamic fibers in lateral spinothalamic tract. These nerve fibers terminate in ventral posterolateral nucleus of thalamus. Some of the fibers terminate in ascending reticular activating system of brainstem.
Slow pain fibers
Fibers of slow pain, which arise from neurons of substantia gelatinosa, cross the midline and run along the fibers of fast pain as paleospinothalamic fibers in lateral spinothalamic tract. One fifth of these fibers terminate in ventral posterolateral nucleus of thalamus. Remaining fibers terminate in any of the following areas:
-
- Nuclei of reticular formation in brainstem
- Tectum of midbrain
- Gray matter surrounding aqueduct of Sylvius.
Third Order Neurons
Third order neurons of pain pathway are the neurons in:
- Thalamic nucleus
- Reticular formation
- Tectum
- Gray matter around aqueduct of Sylvius.
Axons from these neurons reach the sensory area of cerebral cortex. Some fibers from reticular formation reach hypothalamus.
Center for Pain Sensation
Center for pain sensation is in postcentral gyrus of parietal cortex. Fibers reaching hypothalamus are concerned with arousal mechanism due to pain stimulus.
Morphine
Morphine is the principal alkaloid in opium and is widely used till today. Therefore, it is described as prototype.
MOA
Opioid agonists produce analgesia by binding to specific G protein-coupled receptors that are located in brain and spinal cord regions involved in the transmission and modulation of pain. Some effects may be mediated by opioid receptors on peripheral sensory nerve endings.
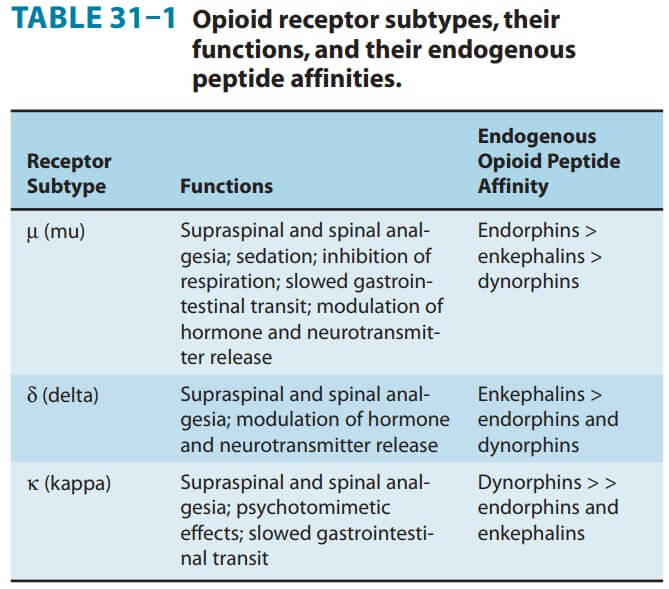
Supraspinal level (medulla) – Block nociceptive transmission binding with receptors in rostroventral region of medulla.
Spinal level- acts on substantia gelatinosa a dorsal horn and decrease excitatory transmitters
Cellular level- At the molecular level, opioid receptors form a family of proteins that physically couple to G proteins and through this interaction affect ion channel gating, modulate intracellular Ca2+ disposition, and alter protein phosphorylation. The opioids have two well- established direct G protein-coupled actions on neurons:
- Close voltage-gated Ca2+ channels on presynaptic nerve terminals and thereby reduce transmitter release- depressed transmitter release—has been demonstrated for release of a large number of neurotransmitters including glutamate, the principal excitatory amino acid released from nociceptive nerve terminals, as well as acetylcholine, norepinephrine, serotonin, and substance P.
- Hyperpolarize and thus inhibit postsynaptic neurons by opening K+ channels.
ACTION
Analgesia:
Morphine and other opioids cause analgesia (relief of pain without the loss of consciousness) and relieve pain both by raising the pain
threshold at the spinal cord level and, more importantly, by altering the brain’s perception of pain. Patients treated with opioids are still aware of the presence of pain, but the sensation is not unpleasant.
Euphoria:
Morphine produces a powerful sense of contentment and well-being. Euphoria may be caused by disinhibition of the dopamine-containing neurons of the ventral tegmental area.
Respiration:
Morphine causes respiratory depression by reduction of the sensitivity of respiratory center neurons to carbon dioxide. This can occur with ordinary doses of morphine in patients who are opioid-naïve and can be accentuated as the dose is increased until ultimately respiration ceases. Respiratory depression is the most common cause of death in acute opioid overdoses. Tolerance to this effect does develop quickly with repeated dosing, which allows the safe use of morphine for the treatment of pain when the dose is correctly titrated.
Depression of cough reflex:
Both morphine and codeine have antitussive properties. In general, cough suppression does not correlate closely with the analgesic and respiratory depressant properties of opioid drugs. The receptors involved in the antitussive action appear to be different from those involved in analgesia.
Miosis:
The pinpoint pupil (Figure 14.8) characteristic of morphine use results from stimulation of μ and κ receptors. There is little tolerance to the effect, and all morphine abusers demonstrate pinpoint pupils. [Note: This is important diagnostically, because many other causes of coma and respiratory depression produce dilation of the pupil.
Emesis:
Morphine directly stimulates the chemoreceptor trigger zone in the area postrema that causes vomiting.
GI tract:
Morphine relieves diarrhea by decreasing the motility and increasing the tone of the intestinal circular smooth muscle. Morphine also increases the tone of the anal sphincter. Overall, morphine and other opioids produce constipation, with little tolerance developing. [Note: A nonprescription laxative combination of the stool softener docusate with the stimulant laxative senna is useful to treat opioid-induced constipation.] Morphine can also increase biliary tract pressure due to contraction of the gallbladder and constriction of the biliary sphincter.
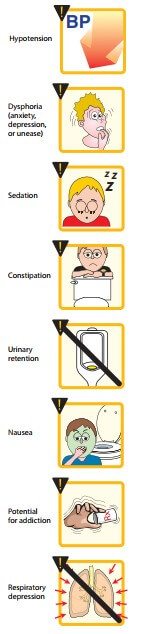
Cardiovascular:
Morphine has no major effects on the blood pressure or heart rate at lower dosages. With large doses, hypotension and bradycardia may occur. Because of respiratory depression and carbon dioxide retention, cerebral vessels dilate and increase cerebrospinal fluid pressure. Therefore, morphine is usually contraindicated in individuals with head trauma or severe brain injury. It also shifts blood from pulmonary to systemic circulation, for this property morphine is used in treatment of left ventricular failure.
ADR
Side effects:
Sedation, mental clouding, lethargy and other subjective effects which may even be dysphoric in some subjects; vomiting is occasional in recumbent patient; constipation is common and distressing. Respiratory depression, blurring of vision, urinary retention (especially in elderly male) are other side effects. BP may fall, especially in hypovolemic patient and if he/she walks about.
Idiosyncrasy and allergy:
Allergic reactions manifesting as urticaria, swelling of lips occur infrequently. Anaphylactoid reaction is rare. A local reaction at injection site and generalized itching may occur due to histamine release.
Apnoea of the newborn:
This may occur when morphine is given to the mother during labour. The blood-brain barrier of the foetus is undeveloped, morphine attains higher concentration in foetal brain than in that of mother. Naloxone 10 µg/kg injected in the umbilical cord is the treatment of choice.
Acute morphine poisoning:
It may be accidental, suicidal or seen in drug abusers. In the nontolerant adult, 50 mg of morphine i.m. produces serious toxicity. The human lethal dose is estimated to be about 250 mg. Manifestations are extensions of the
pharmacological action. Stupor or coma, flaccidity, shallow and occasional breathing, cyanosis, pinpoint pupil, fall in BP and shock; convulsions may be seen in few, pulmonary edema occurs at terminal stages, death is due to respiratory failure.
Treatment: consists of respiratory support (positive pressure respiration also opposes pulmonary edema formation) and maintenance of BP (i.v. fluids, vasoconstrictors). Gastric lavage should be done with pot. permanganate to remove unabsorbed drug. Lavage is indicated even when morphine has been injected. Being a basic drug it is partitioned to the acid gastric juice, ionizes there and does not diffuse back into blood. Specific antidote: Naloxone 0.4–0.8 mg i.v. repeated every 2–3 min till respiration picks up, is the specific antagonist of choice because it acts rapidly, does not have any agonistic action and does not per se depress respiration (see p. 483). Due to short duration of action, naloxone should be repeated every 1–4 hours, according to the response.
Tolerance and dependence:
Treatment: consists of withdrawal of morphine and substitution with oral methadone (long-acting, orally effective) followed by gradual withdrawal of methadone.
Tramadol
This centrally acting analgesic is an atypical opioid which relieves pain by opioid as well as additional mechanisms. Its affinity for µ opioid receptor is low, while that for and and is very low.
Unlike other opioids, it inhibits reuptake of NA and 5-HT, increases 5-HT release, and thus activates monoaminergic spinal inhibition of pain. Its analgesic action is only partially reversed by the opioid antagonist naloxone.
Injected i.v. 100 mg tramadol is equianalgesic to 10 mg i.m. morphine. Oral bioavailability of tramadol is good (oral: parenteral dose ratio is 1.4). The t½ is 5–6 hours and effects last for 4–6 hrs.
Tramadol causes less respiratory depression, sedation, constipation, urinary retention and rise in intrabiliary pressure than morphine.
It is well tolerated; side effects are dizziness, nausea, sleepiness, dry mouth, sweating and lowering of seizure threshold.
Haemodynamic effects are minimal. Tramadol should not be given to patients taking SSRI therapy because of risk of ‘serotonin syndrome’. Tramadol is indicated for mild-to-moderate short-lasting pain due to diagnostic procedures, injury, surgery, etc, as well as for chronic pain including cancer pain, but is not effective in severe pain. Little tendency to dose escalation by chronic users is seen and abuse potential is low.
Dose: 50–100 mg oral/i.m./slow i.v. infusion (children 1–2 mg/ kg) 4–6 hourly.
Pethidine (Meperidine)
Pethidine was synthesized as an atropine substitute in 1939, and has some actions like it. Though chemically unrelated to morphine, it interacts with
µ opioid receptors and its actions are blocked by naloxone. Important differences in comparison to morphine are:
- Dose to dose 1/10th in analgesic potency; however, analgesic efficacy approaches near to morphine and is greater than codeine.
- After i.m. injection, the onset of action is more rapid but duration is shorter (2–3 hours).
- It does not effectively suppress cough.
- Spasmodic action on smooth muscles is less marked—miosis, constipation and urinary retention are less prominent.
- Pethidine is believed to induce less biliary spasm than morphine; traditionally preferred in cholecystitis/biliary colic. However, there is no objective evidence to support this belief. One study* in patients undergoing cholecystectomy found pethidine to raise common bile duct pressure 14% more than equianalgesic dose of morphine.
- It is equally sedative and euphoriant, has similar abuse potential. The degree of respiratory depression seen at equianalgesic doses is equivalent to that with morphine.
- Tachycardia (due to antimuscarinic action) occurs instead of bradycardia.
- It causes less histamine release and is safer in asthmatics.
- It has local anaesthetic action: corneal anaesthesia is seen after systemic doses.
- It is well absorbed, oral: parenteral activity ratio is higher (1/3 to 1/2). Pethidine is nearly completely metabolized in liver. The plasma t½ of pethidine is 2–3 hours. Acidification of urine increases excretion of unchanged pethidine.
SIDE EFFECTS
- These are similar to morphine except those mentioned above.
- Some atropinic effects (dry mouth, blurred vision, and tachycardia) may be noted in addition.
- Overdose of pethidine produces many excitatory effects—tremors, mydriasis, hyperreflexia, delirium, myoclonus and convulsions. This is due to accumulation of norpethidine which has excitant effects.
- Renal failure patients given repeated doses of pethidine are prone to experience similar effects.
- Nonselective MAO inhibitors interfere with hydrolysis but not with demethylation of pethidine—norpethidine is produced in excess and excitement occurs. Pethidine injected in patients receiving a selective serotonin reuptake inhibitor (SSRI) may produce the ‘serotonin syndrome’ (see p. 461) by enhancing 5-HT release.
- Tolerance and physical dependence develop slowly with pethidine. Probably due to its shorter duration of action, body functions get time to recover. For the same reason withdrawal syndrome develops more rapidly.
- Autonomic disturbances are less marked during pethidine withdrawal, than after morphine withdrawal.

USE
Pethidine is primarily used as an analgesic (substitute of morphine) and in preanaesthetic medication, but not for cough or diarrhoea.
- It has also been used to control shivering during recovery from anaesthesia or that attending i.v. infusions. Conventional antihistaminics, NSAIDs and corticosteroids augment this effect of pethidine.
- Potential adverse effects due to accumulation of norpethidine limit its utility in patients who require repeated dosing.
- It is the preferred opioid analgesic during labour, because at equianalgesic doses neonatal respiratory depression is less marked, but still significant.
DOSE
- 50–100 mg i.m., s.c. (may cause irritation, local fibrosis on repeated injection). It is occasionally given orally or i.v. PETHIDINE HCL 100 mg/2 ml inj; 50, 100 mg tab.
USES OF OPOIDS
- Potent analgesic agents (intraoperative/ postoperative)
- Premedication
- Inducing agent
- Blunt cardiovascular response in intubation
- Daycare surgery with remifentanil + Propofol
- Pulmonary edema (morphine)
- Painless labor using epidural analgesia
- Sedatives in ICU
- Abolish postoperative shivering (pethidine, tramadol)