Table of Contents
INSULIN PREPARATIONS

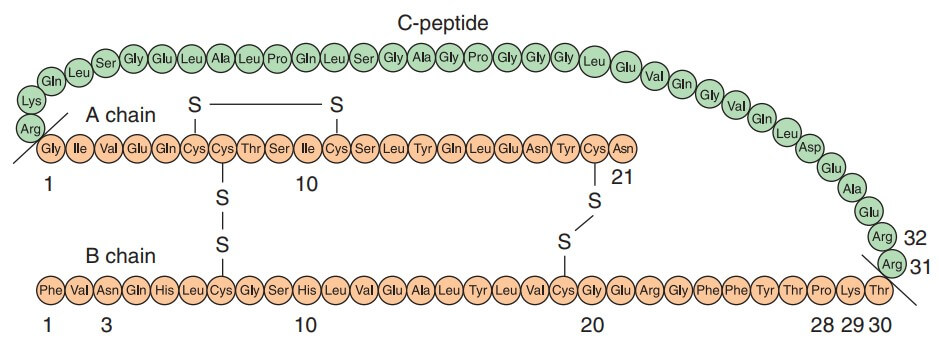
Insulin was discovered in 1921 by Banting and Best who demonstrated the hypoglycemic action of an extract of pancreas prepared after degeneration of the exocrine part due to ligation of pancreatic duct. The chemical structure was fully worked out in 1956 by Sanger. Insulin is a two chain polypeptide having 51 amino acids and MW about 6000.
The A-chain has 21 while B-chain has 30 amino acids. There are minor differences between human, pork and beef insulins: Thus, pork insulin is more homologous to human insulin than is beef insulin.
The A and B chains are held together by two disulfide bonds. Insulin is synthesized in the cells of pancreatic islets as a single chain peptide Preproinsulin (110 AA) from which 24 AAs are first removed to produce Proinsulin. The connecting or ‘C’ peptide (35 AA) is split off by proteolysis in Golgi apparatus; both insulin and C peptide are stored in granules within the cell. The C peptide is secreted in the blood along with insulin.
Regulation of insulin secretion
Under basal condition ~1U insulin is secreted per hour by human pancreas. Much larger quantity is secreted after every meal. Secretion of insulin from B-cells is regulated by chemical, hormonal and neural mechanisms.
CHEMICAL
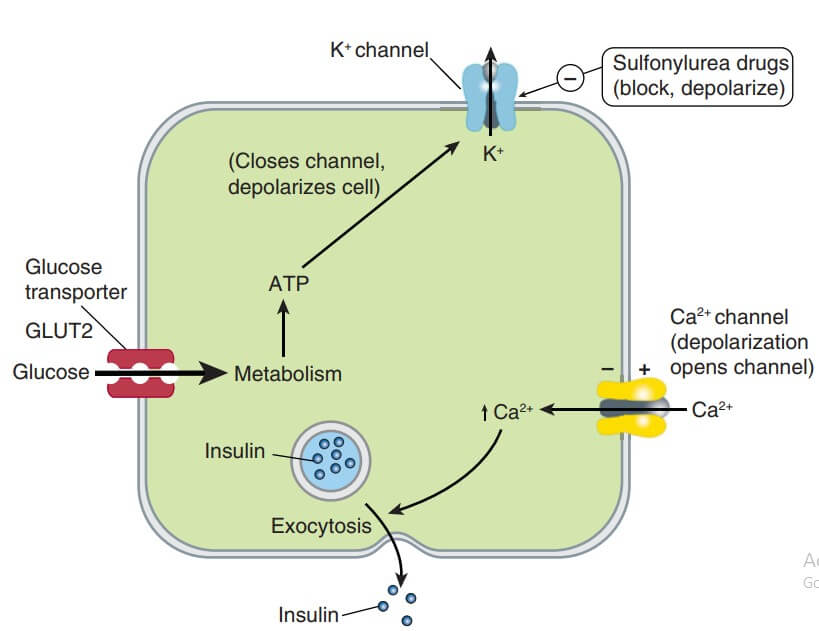
The B- cells have a glucose sensing mechanism dependent on entry of glucose into B cells (through the aegis of a glucose transporter GLUT1) and its phosphorylation by glucokinase. Glucose entry and metabolism leads to activation of the glucosensor which indirectly inhibits the ATP-sensitive K+ channel (K+ATP) resulting in partial depolarization of the cells (see Fig. 19.6). This increases intracellular Ca2+ availability (due to increased influx, decreased efflux and release from intracellular stores) → exocytotic release of insulin storing granules. Other nutrients that can evoke insulin release are—amino acids, fatty acids and ketone bodies, but glucose is the principal regulator and it stimulates synthesis of insulin as well. Glucose induces a brief pulse of insulin output within 2 min (first phase) followed by a delayed but more sustained second phase of insulin release.
HORMONAL
A number of hormones, e.g. growth hormone, corticosteroids, thyroxine modify insulin release in response to glucose. PGE has been shown to inhibit insulin release. More important are the intra-islet paracrine interactions between the hormones produced by different types of islet cells. The B cells constitute the core of the islets and are the most abundant cell type. The B cells, comprising 25% of the islet cell mass, surround the core and secrete glucagon. The B cells (5–10%) elaborating somatostatin are interspersed between the B cells. There are some PP (pancreatic polypeptide containing) cells as well.
- Somatostatin inhibits release of both insulin and glucagon.
- Glucagon evokes release of insulin as well as somatostatin.
- Insulin inhibits glucagon secretion. Amylin, another B cell polypeptide released with insulin, inhibits glucagon secretion through a central site of action in the brain.
The three hormones released from closely situated cells influence each other’s secretion and appear to provide fine tuning of their output in response to metabolic needs.
NEURAL
The islets are richly supplied by sympathetic and vagal nerves.
- Adrenergic α2 receptor activation decreases insulin release (predominant) by inhibiting B cell adenylyl cyclase.
- Adrenergic B2 stimulation increases insulin release (less prominent) by stimulating B cell adenylyl cyclase.
- Cholinergic—muscarinic activation by ACh or vagal stimulation causes insulin secretion through IP3/DAG-increased intracellular Ca2+ in the B cells.
MOA
Insulin acts on specific receptors located on the cell membrane of practically every cell, but their density depends on the cell type: liver and fat cells are very rich. The insulin receptor is a receptor tyrosine kinase (RTK). Binding of insulin to B subunits induces aggregation and internalization of the receptor along with the bound insulin molecules. This activates tyrosine kinase activity of the B subunits → pairs of B subunits phosphorylate tyrosine residues on each other → expose the catalytic site to phosphorylate tyrosine residues of Insulin Receptor Substrate proteins (IRS1, IRS2, etc). In turn, a cascade of phosphorylation and dephosphorylation reactions is set into motion which amplifies the signal and results in stimulation or inhibition of enzymes involved in the rapid metabolic actions of insulin.
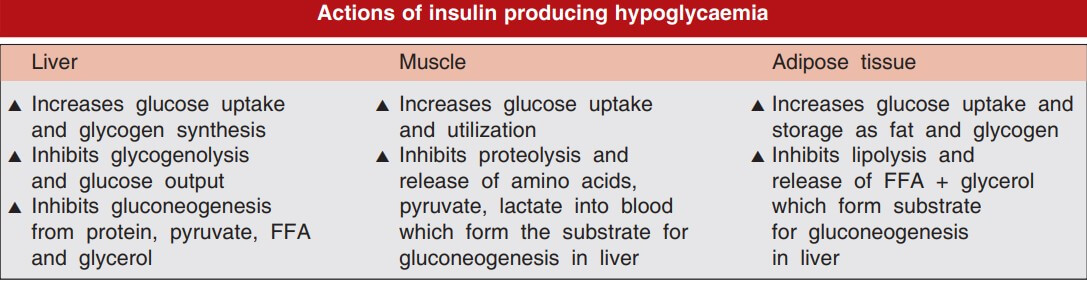
ACTIONS OF INSULIN
REACTIONS TO INSULIN
-
- Hypoglycemia
- Counter regulatory sympathetic hyper reactivity – Tachycardia, palpitations, sweating, tremulousness
- Parasympathetic hyper reactivity– Nausea and hunger
- Deprivation of brain of essential nutrient glucose– Dizziness, headache, visual disturbances, fatigue, weakness, Convulsion or coma
- Insulin allergy- immediate hypersensitivity; due to noninsulin protein contaminant
- Immune insulin resistance– Due to IgG anti-insulin antibody produced during insulin therapy
- Hypoglycemia
Local reactions-
-
-
- Swelling, erythema and stinging
- Lipodystrophy at injection site
- Edema– due to Na retention
- Increased cancer risk– due to insulin resistance and hyperinsulinemia in Type II DM
-
USES OF INSULIN
-
- Diabetes mellitus- Type I, Type II, GDM
Diabetic ketoacidosis (Diabetic coma)
-
- Hyperosmolar non-ketotic hyperglycemia coma (HONK)
- Glucose insulin drip for Hyperkalemia
- Perioperative stress/ infection
Newer insulin delivery devices
INSULIN SYRINGES
Prefilled disposible syringes contain specific types or mixtures of regular and modified insulins.
PEN DEVICES
Fountain pen like: use insulin cartridges for s.c. injection through a needle. Preset amounts (in 2 U increments) are propelled by pushing a plunger; convenient in carrying and injecting.
INHALED INSULIN
An inhaled human insulin preparation was marketed in Europe and the USA, but withdrawn due to risk of pulmonary fibrosis and other complications. The fine powder delivered through a nebulizer controlled mealtime glycaemia, but was not suitable for round-the-clock basal effect. Attempts are being made to overcome the shortcomings.
INSULIN PUMPS
Portable infusion devices connected to a subcutaneously placed cannula—provide ‘continuous subcutaneous insulin infusion’ (CSII). Only regular insulin or a fast acting insulin analogue is used. The pump can be programmed to deliver insulin at a low basal rate (approx. 1 U/hr) and premeal boluses (4– 15 times the basal rate) to control post-prandial glycaemia. Though, theoretically more appealing, no definite advantage of CSII over multidose s.c. injection has been demonstrated. Moreover, cost, strict adherence to diet, exercise, care of the device and cannula, risk of pump failure, site infection, are too demanding on the patient. The CSII may be appropriate for selected type 2 DM cases only.
IMPLANTABLE PUMPS
Consist of an electromechanical mechanism which regulates insulin delivery from a percutaneously refillable reservoir. Mechanical pumps, propellant driven and osmotic pumps have been utilized.
OTHER ROUTES OF INSULIN DELIVERY
Intraperitoneal, oral (by complexing insulin into liposomes or coating it with impermeable polymer) and rectal routes are being tried. These have the advantage of providing higher concentrations in the portal circulation, which is more physiological.