Table of Contents
Classification
BIOSYNTHESIS AND STORAGE OF THYROID HORMONES
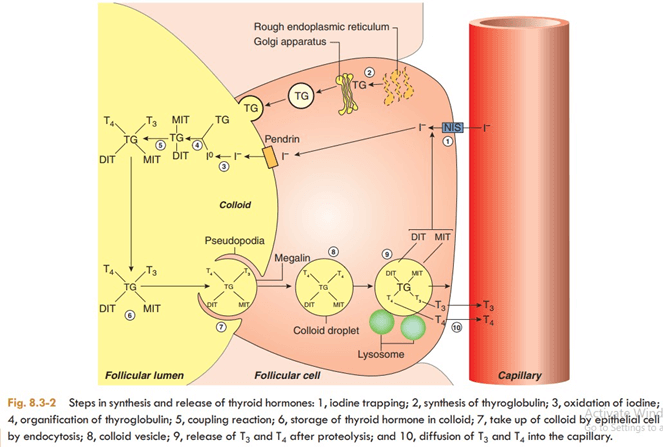
Thyroxine (T4) and triiodothyronine (T3) are synthesized from tyrosine and iodide by the enzyme complex, peroxidase. The steps involved in the synthesis of thyroid hormones are (Fig. 8.3-2):
IODINE TRAPPING
The first step in the synthesis of thyroid hormones is uptake of iodide by the thyroid gland, which occurs against the chemical (about 30:1) and electrical gradients. Therefore I- is absorbed into the thyroid cell by iodide pump or Na/I- symporter. It is an energy requiring process and is linked to the ATPase- dependent Na+-K+ pump.
SYNTHESIS AND SECRETION OF THYROGLOBULIN
Thyroglobulin is a large glycoprotein that is synthesized on the rough endoplasmic reticulum of the thyroid epithelial cells as peptide units of molecular weight 330,000. These units move to the apical plasma membrane and release into the lumen of follicle. Each molecule of thyroglobulin contains about 140 tyrosine residues, which can serve as a substrate for iodine for the formation of thyroid hormones. It is also the storage site of the two hormones within the thyroid gland.
OXIDATION OF IODIDE
Once within the gland, iodide rapidly moves to the apical surface of the epithelial cells. From these, it is transported into the lumen of the follicles by a transporter called pendrin. The iodide (1) is then immediately oxidized to iodine (1° ) by the enzyme peroxidase present near the apical border of the epithelial cells.
ORGANIFICATION OF THYROGLOBULIN
Refers to the iodination of tyrosine residues present in the thyroglobulin molecule. This reaction occurs at the apical membrane of the cell as soon as the thyroglobulin molecule is released by the secretory granules by exocytosis and requires thyroid peroxidase. Tyrosine (of thyroglobulin) is first iodinated at position 3 to form monoiodotyrosine (MIT) and then at position 5 to form diiodotyrosine (DIT).
COUPLING REACTION
Two molecules of DIT couple to form thyroxine (T 4). One molecule of MIT, when coupled with one molecule of DIT, triiodothyronine (T 3) is produced. The enzyme peroxidase is required during coupling.
STORAGE
Once thyroglobulin has been iodinated it is stored in the lumen of the follicle as colloid for several months. It is estimated that the stored thyroid hormones can meet the body requirement for 1- 3 months.
Hormone secretion:
Secretion of the thyroid hormone from the colloid stored in the lumen of follicle involves following steps (Fig. 8.3-2):
ENDOCYTOSIS
The colloid containing iodinated thyroglobulin is retrieved from the lumen of the follicle by the epithelial cells through endocytosis. This process is facilitated by the TG receptor megalin located on the apical membrane. The colloid enters the cytoplasm in the form of colloid droplets by pinocytosis with the formation of reabsorption lacunae, which move through the cytoplasm toward the basal membrane.
PROTEOLYSIS.
The colloid droplets fuse with the lysosome vesicles containing proteolytic enzymes. The proteases digest the thyroglobulin molecule releasing T4, T3, DIT, MIT and other amino acid constituent into the cytoplasm of the epithelial cells. T4 and T3 diffuse through the basal border of epithelial cells into the blood stream via adjacent rich capillary plexus. Monoiodotyrosine and DIT are rapidly deiodinated within the follicular cells by the enzyme deiodinase. In this way, iodide is retrieved for recycling along with the tyrosine into T4 and T3 synthesis.
Transport of T 4 and T 3
Secreted T 4 and T 3 circulate in the blood stream in two forms: bound and free.
BOUND FORM
Most of the circulating T4 (99.95%) and T 3 (99.5%) is bound to specific binding protein.
- Thyroxine binding globulin is the major binding protein which binds about 70% of T 4 and T 3 .
- Thyroxine binding prealbumin binds about 15–20% of T4.
- Thyroxine binding albumin, binds about 10% of the T4.
FREE FORM
Only about 0.05% of T4 and 0.5% T3 circulate unbound (free form) in the plasma. These free, unbound hormones represent the biologically active hormone. Note. It is important to note that most of the circulating T3 is not of thyroid origin but diffuses from the tissues which convert T4 into T3 as described below.
Metabolism and excretion of thyroid hormones The major pathways of peripheral metabolism of circulating thyroid hormone include deiodination, deamination (decarboxylation)
and conjugation with glucuronic acid.
DEIODINATION
In the peripheral tissues, T4 is deiodinated as:
About 40% of T4 is deiodinated into T3 (3,5,3′- triiodothyronine) by the enzyme 5′-deiodinase. Remaining 60% of T4 is deiodinated to reverse T3, i.e. rT3 (3,3′-5′-triiodothyronine) by 5- deiodinase. Reverse T3 is physiologically inert. Note. Since T4 is deiodinated to T3, which produces physiological effects, so T4 itself may be metabiologically inert and hence called a prohormone. T3 and rT3 are deiodinated to DIT (T2) and MIT (T1). DIT (T2) is further deiodinated to MIT (T1).
DECARBOXYLATION
Very small amount of T4 and T3 are metabolised by decarboxylation to form tetraiodothyroacetic acid (TETRAC) and triiodothyroacetic acid (TRIAC).
CONJUGATION
Approximately 15% of thyroid hormones (T4 and T3) are conjugated in the liver to form glucuronides and sulphates. The conjugate then is secreted via the bile duct into the intestine. In normal individuals, metabolites of T4 and T3 are excreted mainly in the faeces with a small amount appearing in the urine.


MOA
Both T3 and T4 penetrate cells by active transport and produce majority of their actions by combining with a nuclear thyroid hormone receptor (TR) which belongs to the steroid and retinoid superfamily of intracellular receptors.
In contrast to the steroid receptor, the TR resides in the nucleus even in the unliganded inactive state. It is bound to the ‘thyroid hormone response element’ (TRE) in the enhancer region of the target genes along with corepressors (Fig. 18.4). This keeps gene transcription suppressed. When T3 binds to the ligand-binding domain of TR, it heterodimerizes with retinoid X receptor (RXR) and undergoes a conformation change releasing the corepressor and binding the coactivator. This induces gene transcription
→ production of specific mRNA and a specific pattern of protein synthesis
→ various metabolic and anatomic effects.
The expression of certain genes is repressed by T3. In their case, the unliganded TR allows gene transcription, while binding of T3 to TR halts the process. Many of the effects, e.g. tachycardia, arrhythmias, raised BP, tremor, hyperglycaemia are mediated, at least partly, by sensitization of adrenergic receptors to catecholamines. Induction of adenylyl cyclase, proliferation of Β adrenoceptors and a better coupling between these two has been demonstrated.
Preparations
These preparations may be synthetic (levothyroxine, liothyronine, liotrix) or of animal origin (desiccated thyroid).
| Levothyroxine (T4) | |
Liothyronine (T3) | Although liothyronine (T3) is three to four times more potent than levothyroxine, it is not recommended for routine replacement therapy because of its shorter half-life (24 hours), which requires multiple daily doses; its higher cost; and the greater difficulty of monitoring its adequacy of replacement by conventional laboratory tests. Furthermore, because of its greater hormone activity and consequent greater risk of cardiotoxicity, T3 should be avoided in patients with cardiac disease. It is best used for short term suppression of TSH. |
| Liotrix (a 4:1 ratio of T4:T3) | Because oral administration of T3 is unnecessary, use of the more expensive mixture of thyroxine andliothyronine (liotrix) instead of levothyroxine is never required. |
Thyroid dessicated (USP) | The use of desiccated thyroid rather than synthetic preparations is never justified, since the disadvantages ofprotein antigenicity, product instability, variable hormone concentrations, and difficulty in laboratory monitoring far outweigh the advantage of lower cost. |
Thyroid hormones are not effective and can be detrimental in the management of obesity, abnormal vaginal bleeding, or depression if thyroid hormone levels are normal.
Synthetic levothyroxine is the preparation of choice for thyroid replacement and suppression therapy because of its stability, content uniformity, low cost, lack of allergenic foreign protein, easy laboratory measurement of serum levels, and long half-life (7 days), which permits once-daily administration. In addition, T4 is converted to T3 intracellularly; thus, administration of T4 produces both hormones. Generic levothyroxine preparations provide comparable efficacy and are more cost-effective than branded preparations.
Significant amounts of T3 found in some thyroid extracts and liotrix may produce significant elevations in T3 levels and toxicity. Equi-effective doses are 100 mg of desiccated thyroid, 100 mcg of levothyroxine, and 37.5 mcg of liothyronine.
The shelf life of synthetic hormone preparations is about 2 years, particularly if they are stored in dark bottles to minimize spontaneous deiodination. The shelf life of desiccated thyroid is not known with certainty, but its potency is better preserved if it is kept dry.
| Drug | Dosage | Action | ADR | USES |
Thyroxine 25, 50,100, 200mcg tablets | (3-5mcg/kg body wt.) | Growth and development- metamorphosis of frog; Control of protein synthesis Metabolism Lipid- enhance lipolysis Carbohydrate- increased utilization of sugar by tissue; glycogenolysis and gluconeogenesis Protein- synthesis of certain protein increase but overall action is catabolic Calorigenesis- Increase in BMR; important for maintaining body temperature CVS- Stimulate rate and force of contraction of myocardium; A fib., angina common in hyperthyroidism CNS- MR in cretinism; nervousness, anxiety in hyperthyroidism Skeletal muscle- Myxedema- Flabby and weak Thyrotoxicosis- increased tone, tremor and weakness GIT- propulsive activity increase Haemopoiesis- facilitatory of erythropoiesis; hypothyroid individual suffer from anemia Reproduction- fertility impaired in hypo | Diarrhea Wt. loss Palpitations Angina Tremors, hyperkinesia Irritability Nervousness Insomnia | Cretinism Adult Hypothyroidism Myxoedema coma- emergency; characterized by progressive mental deterioration due to acute hypothyroidism TSH dependent Nontoxic goiter Thyroid nodule- TSH suppressed which may help to regress benign functioning nodules Papillary carcinoma of thyroid- T4 suppresses TSH production resulting in temporary regression Empirical use- Refractory anemia, Mental depression, Menstrual disorders, Infertility not corrected by usual treatment, Chronic non-healing ulcer, Obstinate constipation |
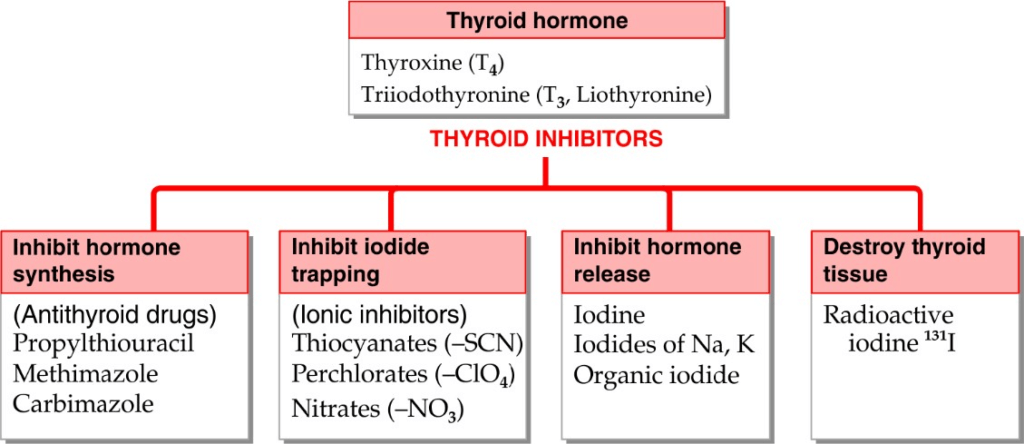
THYROID INHIBITORS
| Drug | Dosage | MOA | ADR | USES |
Thioamides: Methimazole Carbimazole Propylthiouracil | PTU– upto 600 mg qds.Methimazole– 5-20 mg/dayCarbimazole– 30-60 mg initially, 5-20 mg. later | Bind to thyroid peroxidase and prevent oxidation of iodide/ iodotyrosyl residues, thereby: Inhibit iodination of tyrosine residues in thyroglobulin Inhibit coupling of iodotyrosine residue to form T3, T4 PTU- also inhibit peripheral conversion of T4 to T3 by D1 type of 5’DI, but not DS; Methimazole and carbimazole do not have this action Carbimazole acts by getting converted to methimazsdole in body and is longer acting than PTU | Overtreatment- Hypothyroidism and goiter; reverse on stopping drug GI intolerance, Nausea Altered taste or smell- methimazole Maculopapular rash, fever Loss or graying of hair Severe hepatitis; liver damage- PTU Cholestatic jaundice- methimazole Agranulocytosis- (granulocyte <500 cells/mm3)- give antibiotics and stop the drugs | Definitive therapy– Thyrotoxicosis- Graves ds.; toxic multinodular goiter Preoperatively- surgery in thyrotoxic patient Along with I-131- to make pt to euthyroid before starting radioiodine |
Ionic inhibitors | Toxic not used clinically | Monovalent anions– inhibit iodide trapping by NIS into thyroid probably because of similar hydrated ionic size | Thiocyanates- Liver, kidney, BM and brain toxicity Perchlorates- rashes, fever, aplastic anemia, agranulocytosis | Not used clinically |
Iodine and Iodides | Sod. Or potassium 6-10 mg/day | It is fastest acting thyroid inhibitor. Inhibition of hormone release- “thyroid constipation”. Endocytosis of colloid and proteolysis of thyroglobulin comes to halt Excess iodine inhibits its own transport to thyroid cell by interfering with expression of NIS on CM Excess iodine interfere with organification- Wolff- Chaikoff effect | Acute reaction– in person sensitive to iodine, can be triggered with minute quantity. Swelling of lips, eyelids Angioedema of larynx Fever Joint pain Petechial hemorrhage Lymphadenopathy Chronic overdose (iodism) Inflammation of mucous membrane Salivation Rhinorrhea Sneezing Lacrimation Swelling of eyelid Burning sensation in mouth Headache Rash GI symptoms Precipitate thyrotoxicosis in MN goitre | Preoperative preparation- for thyroidectomy in Grave’s ds.- make gland firm, less vascular and easier to operate on Thyroid storm- Lugol’s iodine Prophylaxis of endemic goiter Antiseptic- Tincture of iodine, povidone iodine |
Radioactive iodine: I-131 | 5-10 mcg on empty stomach Dissolved in water and taken orally | I-131 is concentrated by gland Emits X-rays as well as B- particles Follicular cell undergo pyknosis and necrosis followed by fibrosis | Hypothyroidism: 5-10% of pts. Genetic damage Thyroid carcinoma Long latent period of response Contraindicated in pregnancy- damage to fetal tissue Not suitable for young patient | Diagnostic- thyroid scanning; X- Ray measured Hyperthyroidism- Graves’ ds., Toxic nodular goiter Metastatic carcinoma of thyroid- for palliative care after thyroidectomy |
| Carbimazole is 5 times potent than PTU. PTU need multiple doses due to less half-life. Carbimazole convert to active metabolite- methimazole. | ||||