Table of Contents
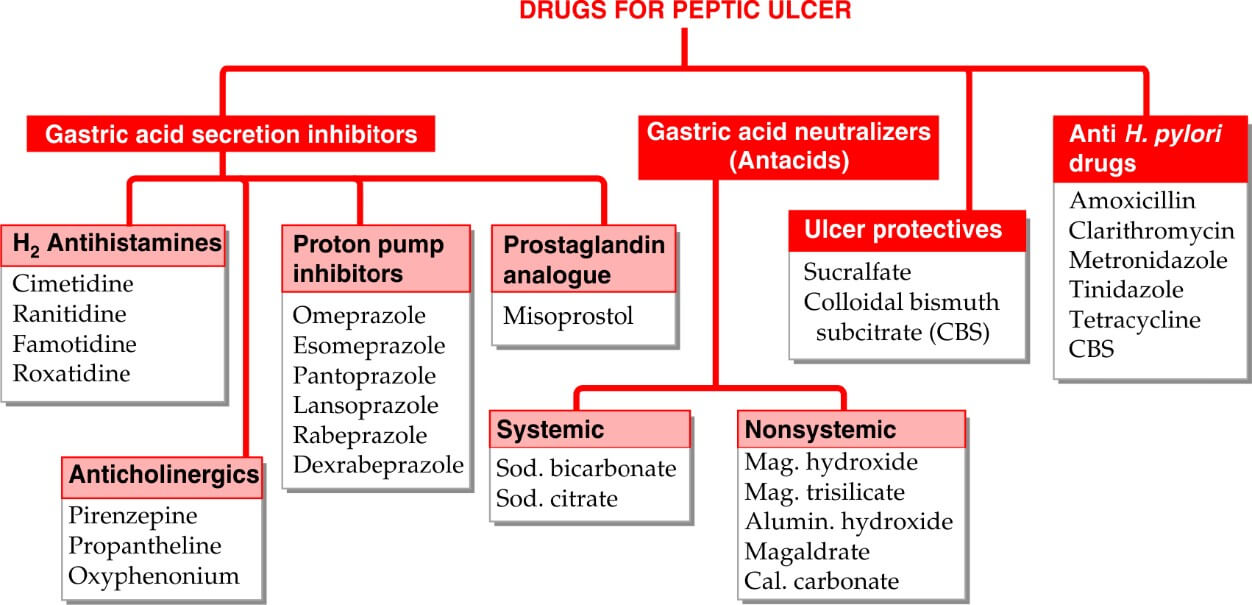
Classification:
|
Drug |
Dosage |
MOA |
ADR |
USES |
|
H2-Antagonist (Ranitidine) |
|
|
|
|
Proton pump inhibitors:
DOSE- Omeprazole-20 mg OD; Pantoprazole- 40 mg OD
MOA
- Inhibit final common step in gastric acid secretion
- PPI have overtaken H2 blocker for acid-peptic diorders
- Only significant pharmacological action of omeprazole is dose dependent suppression of gastric acid secretion; without anticholinergic or H2 blocking action
- It is a powerful inhibitor of gastric acid: can totally abolish HCl secretion, both resting as well as that stimulated by food or any of the secretagogues, without much effect on pepsin, intrinsic factor, juice volume and gastric motility
- Omeprazole is inactive at neutral pH, but at pH < 5 it rearranges to two charged cationic forms (a sulphenic acid and a sulphenamide configurations) that react covalently with SH groups of the H+K+ATPase enzyme and inactivate it irreversibly, especially when two molecules of omeprazole react with one molecule of the enzyme.
- After absorption into bloodstream and subsequent diffusion into the parietal cell, it gets concentrated in the acidic pH of the canaliculi because the charged forms generated there are unable to diffuse back.
- Moreover, it gets tightly bound to the enzyme by covalent bonds. These features and the specific localization of H+K+ATPase to the apical membrane of the parietal cells confer high degree of selectivity of action to omeprazole.
- Acid secretion resumes only when new H+K+ATPase molecules are synthesized (reactivation half time 18 hours).
- It also inhibits gastric mucosal carbonic anhydrase
|
ADR |
USES |
|
|
ANTACIDS:
These are basic substances which neutralize gastric acid and raise pH of gastric contents. Peptic activity is indirectly reduced if the pH rises above 4, because pepsin is secreted as a complex with an inhibitory terminal moiety that dissociates below pH 5: optimum peptic activity is exerted between pH 2 to 4.
Antacids do not decrease acid production; rather, agents that raise the antral pH to > 4 evoke reflex gastrin release → more acid is secreted, especially in patients with hyperacidity and duodenal ulcer; “acid rebound” occurs and gastric motility is increased.
The potency of an antacid is generally expressed in terms of its acid neutralizing capacity (ANC).
Systemic Antacids:
SODIUM BICARBONATE
It is water soluble, acts instantaneously, but the duration of action is short. It is a potent neutralizer (1 g → 12 mEq HCl), pH may rise above 7. However, it has several demerits:
- Absorbed systemically: large doses will induce alkalosis.
- Produces CO2 in stomach → distention, discomfort, belching, and risk of ulcer perforation.
- Acid rebound occurs, but is usually short lasting.
- Increases Na+ load: may worsen edema and CHF.
Use of sod. bicarbonate is restricted to casual treatment of heartburn. It provides quick symptomatic relief. Other uses are to alkalinize urine and to treat acidosis.
Uses of sodium bicarbonate
- Systemic antacid
- Metabolic acidosis
- To render urine alkaline in UTI or to prevent precipitation of sulfonamide or uric acid
- Locally: Antipruritic lotion, for mouth or eye wash
SODIUM CITRATE-
similar to sod. bicarbonate; 1 g neutralizes 10 mEq HCl; CO2 is not evolved.
Non-systemic Antacids
These are insoluble and poorly absorbed basic compounds; react in stomach to form the corresponding chloride salt. The chloride salt again reacts with the intestinal bicarbonate so that HCO¯3 is not spared for absorption—no acid-base disturbance occurs. However, small amounts that are absorbed have the same alkalinizing effect as NaHCO3.
MAG. HYDROXIDE:
Has low water solubility: its aqueous suspension (milk of magnesia) has low concentration of OH¯ ions and thus low alkalinity. However, it reacts with HCl promptly and is an efficacious antacid (1 g → 30 mEq HCl). Rebound acidity is mild and brief.
MILK OF MAGNESIA 0.4 g/5 ml suspension: 5 ml neutralizes 12 mEq acid.
MAGNESIUM TRISILICATE:
Has low solubility and reactivity; 1 g can react with 10 mEq acid, but in clinical use only about 1 mEq is neutralized. About 5% of administered Mg is absorbed systemically—may cause problem if renal function is inadequate. All Mg salts have a laxative action by generating osmotically active MgCl2 in the stomach and through Mg2+ ion induced cholecystokinin release. Soluble Mg salts are used as osmotic purgatives.
ALUMINIUM HYDROXIDE GEL:
It is a bland, weak and slowly reacting antacid. On keeping it slowly polymerizes to variable extents into still less reactive forms. Thus, the ANC of a preparation gradually declines on storage. Also, the product from different manufacturers may have differing ANCs; usually it varies from 1–2.5 mEq/g. Thus, 5 ml of its suspension may neutralize just 1 mEq HCl. As such, little worthwhile acid neutralization is obtained at conventional doses. The Al3+ ions relax smooth muscle. Thus, it delays gastric emptying. Alum. hydrox. Frequently, causes constipation due to its smooth muscle relaxant and mucosal astringent action. Alum. hydrox. Binds phosphate in the intestine and prevents its absorption—hypophosphatemia occurs on regular use. This may:
- cause osteomalacia
- Be used therapeutically in hyperphosphatemia and phosphate stones.
Small amount of Al3+ that is absorbed is excreted by kidney. This is impaired in renal failure—aluminum toxicity (encephalopathy, osteoporosis) can occur.
ANTACID COMBINATIONS
- A combination of two or more antacids is frequently used. These may be superior to any single agent on the following accounts:
- Fast (Mag. hydrox.) and slow (Alum. hydrox.) acting components yield prompt as well as sustained effect.
- Mag. salts are laxative, while alum. Salts are constipating: combination may annul each other’s action and bowel movement may be least affected.
- Gastric emptying is least affected; while alum. Salts tend to delay it, mag./cal. salts tend to hasten it.
- Dose of individual components is reduced; systemic toxicity (dependent on fractional absorption) is minimized.
Uses of antacids
Antacids are no longer used for healing peptic ulcer, because they are needed in large and frequent doses, are inconvenient, can cause acid rebound and bowel upset, afford little nocturnal protection and have poor patient acceptability. Antacids are now employed only for intercurrent pain relief and acidity, mostly self-prescribed by the patients as over the counter preparations. They continue to be used for non-ulcer dyspepsia and minor episodes of heartburn.
GASTROESOPHAGEAL REFLUX
Antacids afford faster symptom relief than drugs which inhibit acid secretion, but do not provide sustained benefit. May be used off and on for acid eructation and heartburn.